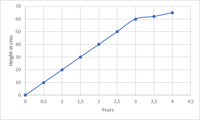
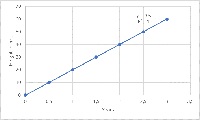
What is the ICH Q2(R2) guideline?

“Validation of Analytical Procedures” (ICH Q2(R2)) published by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human (ICH) is a tripartite harmonized guideline that identifies validation parameters needed for variety of laboratory methods to analyze drugs. The three parties involved in this harmonization are the US, EU and Japan. There are quite a lot of other ICH guidelines, which are categorized into 4 major groups: