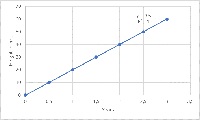
What is the range of an analytical method?
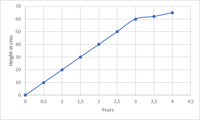
In this article we will have a short look on the range of a method in context of analytical method validation.
The common guideline used for method validation, the ICH Q2(R1), defines range as an interval from the upper to the lower concentration of the analyte in the sample e.g. drugs for which the analytical method has been demonstrated to work with acceptable level of trueness, precision, and linearity. The linearity studies for a method usually define the range for it.