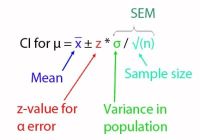
Template method validation plan assay

After we’ve already provided a template of a method validation plan for an identification method, we now like to share a template for a validation plan for an assay. Assays include content and potency determinations.