Template method validation plan assay
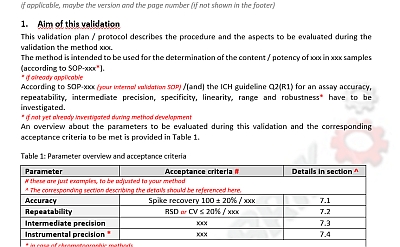
After we’ve already provided a template of a method validation plan for an identification method, we now like to share a template for a validation plan for an assay. Assays include content and potency determinations.
What is such a template used for?
In the pharmaceutical area, all analytical methods used to test drugs (drug substances and drug products) must be validated before they are allowed to be used for the first time. This is to demonstrate that they actually fulfill their intended purpose in a satisfactory and reliable manner. Therefore, you perform an analytical method validation, which must be described in a plan that covers all regulatory required validation parameters, such as trueness and precision (to name just 2). A template for such a plan can be extremely useful.
Template method validation plan assay
Further templates for impurity tests and an overall validation plan will follow soon.
