Template method validation plan identification test
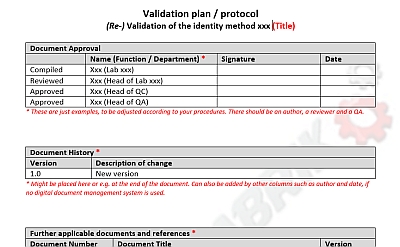
In case a method (that is intended to be used for quality control of drugs in the future) is to be (re-) validated in a pharmaceutical laboratory, a plan for method validation is required. The regulatory requirements such as the ICH Q2(R1) must be taken into account. A clearly structured and understandable format of a validation plan facilitates the execution of the validation of the analytical method.
An example of a method validation plan for an identification method is provided here. This gives insights into possible documentation and can be adapted according to your company's internal requirements.
Template method validation plan for an identity test
Further templates for impurity tests and assays (for content determination and potency) will follow shortly.
