Influence of polysorbate 20 on the filter integrity test of a PVDF sterile filter
As part of the release testing of parenteral pharmaceuticals, sterility of the pharmaceuticals must be ensured after filling. Since a direct check of every filled vial or every syringe cannot be performed, the sterile filters are checked for their integrity, more precisely for their pore diameter, instead. According to the specifications of the WHO, a pore diameter of maximum 0.2 µm is allowed for sterile filtration [1]; accordingly, filtration with a sterile filter possessing a pore diameter of 0.2 µm or less results in a sterile filtrate.
The pore diameter of a sterile filter is checked applying so-called filter integrity tests. An example of such a filter integrity test is the bubble point test. The bubble point of a filter is product-specific and must be previously determined during filter validation. Then, the determined bubble point value is set as an integrity criterion for the corresponding sterile filter for the filtration of this pharmaceutical, and it is used to indirectly assess the sterility of the filtered solution. A measured bubble point pressure above or equal to the integrity criterion proves successful sterile filtration.
The bubble point measurement is based on the capillary behavior of the filter membrane’s nanopores [2]. According to the theory of capillary ascent, the pores of a liquid-wetted filter fill up without the influence of other external forces. The liquid’s increase in the respective pore depends on the capillary diameter, the wetting angle between the liquid and the solid phase and the surface tension of the liquid [3]. The counterpressure required to force the liquid out of the pores must be appropriately higher than the forces acting within the capillaries. This pressure is called the bubble point.
A disadvantage of the bubble point method is the assumption of constant product solution and filter properties. However, when the highly complex pharmaceutical product solutions interact with the filter membrane, changes in the surface properties of the filter or the properties of the liquid can occur. This can confound the bubble point pressure, which can lead to problems regarding the release the filtered drug product batch. A frequently used excipient in pharmaceuticals is polysorbate 20. It has the potential to change the bubble point pressure, as will be explained below. Polysorbate 20 is an amphiphilic molecule that can adsorb to the surface of PVDF sterile filters [4]. Amphiphilic molecules can reduce the surface tension of a solution. Since the bubble point is a function of the surface tension, it can be assumed that adsorption of the amphiphilic molecule polysorbate 20 to the filter membrane could result in a change of the bubble point. This hypothesis was examined and proven in a bachelor thesis.
Experimental design
To generate the results, a placebo solution (same composition as the drug, but without active pharmaceutical ingredient) containing polysorbate 20 was circulated through a PVDF sterile filter. Samples were taken from a collecting container at specific time points over a period of 48 hours and their polysorbate 20 concentration was determined. In parallel, the bubble point pressure was measured. In addition, the concentrations of various individual polysorbate components (various free fatty acids) were investigated. The experiment was performed four times in total.
Results and discussion
An exponential decrease with characteristics that were similar over time was observed in all experiments both for the bubble point and for the polysorbate 20 concentration. Figure 1 shows exemplarily the progress of both measured variables from one of the experiments.
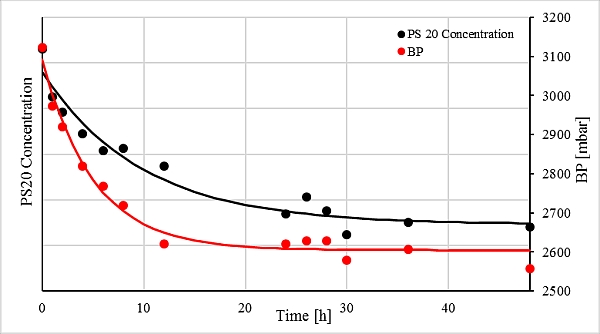
Figure 1: Progress of measured bubble point values and polysorbate 20 concentration. Due to confidentiality obligations, the scaling of the y-axis is not shown.
Progress of the polysorbate 20 concentration
The reduction of the measured polysorbate 20 concentrations can be attributed to the adsorption to the filter [4,5]. Adsorption removes polysorbate 20 from solution. An exponential decrease is quite typical for the adsorption of molecules to a solid. It is an equilibrium reaction that consists of two reactions: adsorption and desorption. Initially, the binding sites of the filter are unoccupied and all polysorbate 20 molecules are in solution. The reaction towards the side of the adsorbed particles predominates strongly, which leads to a sharp decrease in the polysorbate 20 concentration. The more molecules occupy the binding sites of the filter; the more particles desorb. The decrease in concentration becomes smaller and approaches a limit value. Above this limit, the same number of molecules desorb and adsorb. The eponymous equilibrium of the reaction has been reached.
Influence of polysorbate 20 on the bubble point
Analogous to the exponential decrease in the polysorbate 20 concentration, the bubble point values also decreased. According to this observation, the bubble point value is dependent on the amount of adsorbed polysorbate 20 molecules. As the adsorption of polysorbate 20 to the filter surface increases, the bubble point decreases. But what is the underlying mechanism? According to the Laplace-Young equation, three causes result in decreased bubble point pressures:
- a reduction in surface tension,
- an increase in the wetting angle and
- an enlarged pore diameter.
When substances are adsorbed within a pore, the pore diameter decreases, so an increase in the pore diameter can be excluded as underlying cause. According to Zhou et. al. [4] the polysorbate 20 adsorption is of hydrophobic nature, i.e. the polar heads of polysorbate 20 are presented to the aqueous medium by hydrophobic attachment to the filter membrane. Such a polarization of the surface lowers the wetting angle. Correspondingly, this can also be excluded as underlying cause and the observed decrease in the bubble point related to polysorbate 20 adsorption must therefore be due to a reduction in the surface tension.
As already mentioned at the beginning, amphiphilic molecules such as polysorbate 20 possess the property of reducing the surface tension of solutions due to their structure. The polysorbate 20 concentration therefore significantly determines the surface tension of the solution. An increased polysorbate 20 concentration leads to a reduced surface tension, provided that the amphiphilic molecules are in solution. In case of adsorption to the filter surface, however, the polysorbate 20 molecules are removed from the solution. The observed reduced bubble point values must therefore be based on a further phenomenon which has led to an accumulation of dissolved polysorbate 20 in the vicinity of the filter.
One possibility of accumulation would be a retention of dissolved polysorbate 20 molecules in the vicinity of the filter. According to Nayem et. al. [6] the largest polysorbate 20 micelles develop with a radius of gyration of 2.94 nm. However, the radius of the sterile filter is 100 nm and therefore enables the micelles to pass through the membrane. Accordingly, mechanical retention is impossible. Accumulation through unknown interactions of individual polysorbate 20 components after filter saturation can also be excluded. The results of Mahler et. al. [7] show that the composition of polysorbate does not change after filtration using polysorbate 20-saturated PVDF sterile filters. In case of retention caused by a wide variety of interactions, however, the polysorbate 20 composition must have changed due to the different interactions and affinities of the individual components with the saturated filter. This was not observed during this work, as no changes in the concentrations of the free fatty acids investigated were found.
The bubble point measurement method has a further influence on the filter. Due to the pressurization with non-polar nitrogen, it is thermodynamically unfavorable for the adsorbed polysorbate 20 molecules to be hydrophobically adsorbed to the filter, since the polar heads would be directed to the non-polar gas phase. Instead, they desorb from the filter surface and dissolve. The concentration of free polysorbate 20 molecules in the pores strongly increases. The local increase in concentration inside the pore in turn leads to a reduced surface tension. The effect is increased by the number of adsorbed polysorbate 20 molecules. This means that the lowest bubble point pressures are achieved with the maximum number of adsorbed polysorbate 20 molecules. As side effect, the desorption of the polysorbate 20 molecules restores the natural wetting angle and the pore diameter of the filter. The consequence is the observed decrease in the bubble point.
Conclusion
The influence of adsorbed polysorbate 20 on the bubble point value could be proven by the results. The underlying mechanism remains largely unexplained but could be due to the interplay between adsorption and desorption. This hypothesis needs to be checked in further work.
References
[1] WHO (2011). Annex 6 WHO good manufacturing practices for sterile pharmaceutical products, Technical Report Series, 961:261-284.
[2] Jornitz M.W., Agalloco J.P., Akers J.E., Madsen R.E., Meltzer T.H. (2001). Filter Integrity Testing in Liquid Applications, Revisited, Pharmaceutical Technology, Vol. 25 (10):34–50
[3] Jornitz M.W. (2006). Integrity Testing. in Scheper T., Jornitz M.W., Sterile Filtration: Advances in biochemical engineering / biotechnology, Vol. 98: 143–180.
[4] Zhou J. X., Qiu J., Jiang G., Zhou C., Bingham N., Yeung H., Dransart B., Wadhwa M-V., Tressel T. (2008). Non-specific binding and saturation of Polysorbate-20 with aseptic filter membranes for drug substance and drug product during mAb production, Journal of Membrane Science, Vol. 325 (2):735–741.
[5] Lei M., Sugahara J., Hewitt D., Beane D., Jayakar R., Cornell C., Skidmore K., Kao Y.H., Ji J. (2013). The effects of membrane filters used in biopharmaceutical processes on the concentration and composition of polysorbate 20, Biotechnology progress, Vol. 29 (6):1503–1511.
[6] Nayem J., Zhang Z., Tomlinson A., Zarraga I.E., Wagner N.J., Liu Y. (2020). Micellar Morphology of Polysorbate 20 and 80 and Their Ester Fractions in Solution via Small-Angle Neutron Scattering, Journal of Pharmaceutical Sciences, Vol. 109 (4):1498–1508.
[7] Mahler H.C., Huber F., Kishore R.S., Reindl J., Rückert P., Müller R. (2010). Adsorption behavior of a surfactant and a monoclonal antibody to sterilizing-grade filters, Journal of Pharmaceutical Sciences, Vol. 99 (6):2620–2627.
About the author
|
|
Kilian Rauscher studied biotechnology at Darmstadt’s University of Applied Sciences, completed an internship of several months focused on disposables in the pharmaceutical industry and, as part of his bachelor thesis, dealt with the influence of polysorbate 20 on the filter integrity test of a PVDF sterile filter. He will continue his studies in the coming semester with a master’s program of molecular biotechnology at the Technical University of Munich. |

