Multiple use of sterile filters?

Some time ago, I received a very interesting request that I’d like to use as an opportunity for writing this blog article.
The question
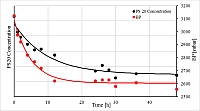
According to the draft of the revised Annex 1 "Manufacture of Sterile Medicinal Products" of the EU GMP guidelines, sterile filters should be discarded after having been used. Multiple use is excluded, with the exception that a use for more than one working day has been validated. This phrasing is not quite unambiguous. So how should we interpret the following requirement "Liquid sterilizing filters should be discarded after the processing of a single lot. The same filter should not be used for more than one working day unless such use has been validated." (8.89)?