What is qualification?
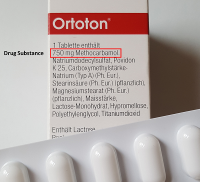
Since laboratory equipment is used when performing analytical methods in the quality control laboratories of pharmaceutical companies or contract laboratories, it must be demonstrably suitable and may not, for example, be used "just like that" (i.e. without prior verification) as in the academic environment. Today, we’d like to deal with this demonstrable suitability.
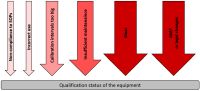
The purpose of Analytical Instrument Qualification (AIQ) is to demonstrate the suitability of the instrument for its intended use. It is not just a single step process, but a collection of different processes in which many documents, the so-called qualification documents, are created. Depending on the device, this process includes not only the basic check of the function(s) of the device but also, for example, safety aspects for the operator. A very good description is provided in the United States Pharmacopoeia (USP) chapter <1058>. Further requirements can also be found, for example, in Annex 15 of the EU GMP Guidelines. AIQ is mainly comprised of 4 categories: