What is qualification?
Since laboratory equipment is used when performing analytical methods in the quality control laboratories of pharmaceutical companies or contract laboratories, it must be demonstrably suitable and may not, for example, be used "just like that" (i.e. without prior verification) as in the academic environment. Today, we’d like to deal with this demonstrable suitability.
The purpose of Analytical Instrument Qualification (AIQ) is to demonstrate the suitability of the instrument for its intended use. It is not just a single step process, but a collection of different processes in which many documents, the so-called qualification documents, are created. Depending on the device, this process includes not only the basic check of the function(s) of the device but also, for example, safety aspects for the operator. A very good description is provided in the United States Pharmacopoeia (USP) chapter <1058>. Further requirements can also be found, for example, in Annex 15 of the EU GMP Guidelines. AIQ is mainly comprised of 4 categories:
1. Design Qualification (DQ) = "Definition of the requirements"
Design qualification (DQ) is the documented collection of activities that define the functional and operational specifications of the instrument and criteria for selection of the vendor, based on the intended purpose of the instrument. It can be regarded as the planning phase of the requirements to be applied for a specific instrument. DQ should be performed both by the developer and the user. The developer must design and manufacture the instrument as per the user requirements specification (URS), if possible, in a quality control environment. The user on the other hand, can justify the selection of the vendor based on method selection and intended use of the instrument and the compliance of the vendor to the specifications set by the user. DQ ensures that instruments have all the necessary functions that can be successfully implemented for the intended purpose. DQ makes sense for big instruments or machines used in production, for a simple microscope it might be a little bit too much ;-)
2. Installation Qualification (IQ)
Installation qualification (IQ) is the documented collection of activities necessary to establish that an instrument is delivered as designed and specified (by a comparison of the URS against the reality), is properly installed in the selected environment, and that this environment is suitable for the instrument. IQ allows user responsibility to ensure proper installation of the instrument with direct support from the vendor. IQ requires the user to establish and maintaining appropriate working environment, but on the other side it allows the identification of any instrumental damage or any variation from the placed order. Let’s illustrate this with an example. Imagine a lab where a new fluorescence microscope has been bought. During IQ, the microscope is situated at its predefined place, all cables are connected, the software is installed, all documents like e.g. the user manual are handed over and it is switched on for the first time.
3. Operational Qualification (OQ)
Operational Qualification (OQ) is a documented collection of activities required to demonstrate that an instrument will generally function according to its operational specification in a defined environment. Or to put it more simply, the OQ provides an answer to the question of whether the instrument is working in my lab exactly as I expected it to do. According to the USP, “Users, or their qualified designees, should perform these tests to verify that the instrument meets manufacturer or user specifications in the user’s environment. Designees could be, for example, vendor representatives.” It is important to test the instrument at the user’s defined environment because mechanical damage is possible during transport and installation that may affect the performance of the instrument. This step can also include a first calibration of the instrument and a training by the vendor of the personal which will work with the new instrument. For instruments with built-in sensors such as e.g. temperature sensors of incubators, calibration of these sensors is part of the OQ. For our microscope example, in OQ the general ability to visualize fluorescent staining is checked, e.g. by checking a slide with fixed cells stained with Cyber green. This is some kind of unspecific and not yet related to any routine test.
4. Performance Qualification (PQ)
Performance Qualification (PQ) is the documented collection of activities necessary to demonstrate that an instrument consistently performs also under routine conditions according to the specifications defined by the user and is thus indeed suitable for the intended use. Although, the PQ and OQ appear very similar, there are distinct differences between the two. PQ is performed more frequently than OQ and is done under similar conditions that are for routine analysis. In our microscope example, in the PQ a routine test like the comet assay to detect DNA damage for which previously silver stained samples have been used is now performed and analysed applying fluorescence staining to be visualized using the new microscope. Using a sample with known damage (already analysed with silver staining), can the new microscope be proven to show the same result? Recording the temperature profile of a maximally loaded autoclave is another PQ example.
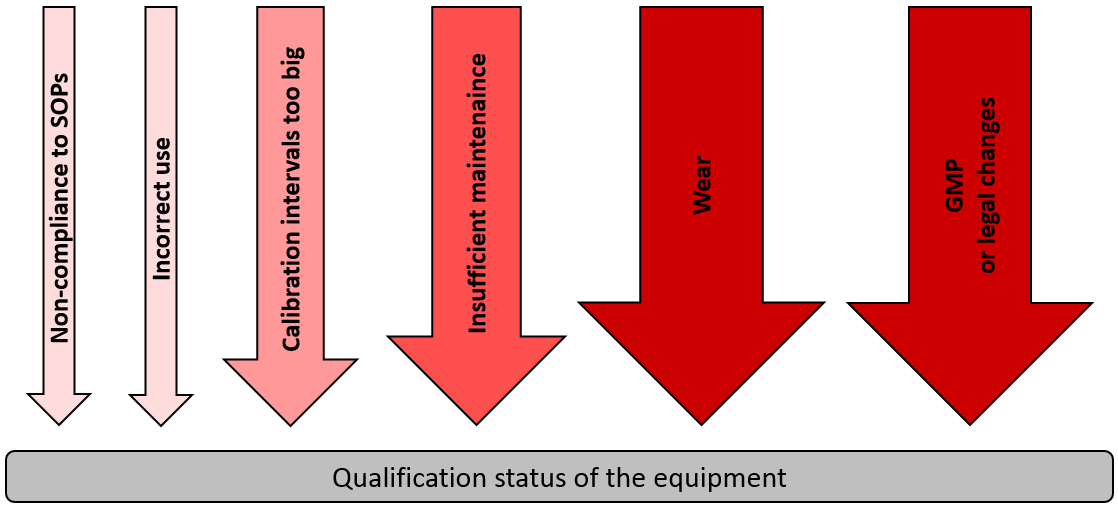
To enable the laboratory personnel to recognize immediately that the laboratory device has been released for use after passing the initial qualification, a green sticker on the device, for example, can be used as visual identifier. In addition, a device logbook should accompany the life cycle of the device. After a successful initial qualification, however, there will always be moments in the life cycle of the device in which a requalification is necessary. The following figure illustrates some of the influencing factors.
** Speaking of incubators: A short anecdote: During transfers, please ensure that qualification is also performed at all sites with uniform specifications ensuring that no incubator was qualified with 37°C ± 1°C at one site, while it may have 37°C ± 2°C at another site. At first glance, this may not seem to cause any problems, but if subsequently, a global method is rolled out across all sites and the site with ± 1°C has already submitted its method during market authorization application, the site with ± 2°C is left behind and may have to re-qualify...
