The Horwitz function and its application in method validations
During a recent method validation, I came across the “Horwitz function,” which shows a correlation between analyte concentration and observed measurement precision in analytical methods. In this blog post, I’d like to discuss its significance in some more detail.
A little history and background
This empirical observation dates back to studies published in 1980 and 1982 by William Horwitz, a renowned former statistician of the FDA [1, 2]. An evaluation of over 150 independent AOAC interlaboratory collaborative studies involving a wide variety of laboratories and analytical methods for different areas of application (pharmaceuticals, but mainly food analysis) has shown that there is a kind of “basic curve” of analytical precision: If the concentration C of an analyte decreases by a factor of 100, the relative standard deviation of reproducibility (RSDR) doubles by about two orders of magnitude [2]. Put more simply: if the RSDR is e.g. 2% at 100% analyte concentration, it is 4% at 1% analyte concentration, 8% at 0.01% analyte concentration, and so on…
Although we should rather talk about mass fraction than analyte concentration...
This pattern is called the “Horwitz trumpet” because it looks like a funnel:
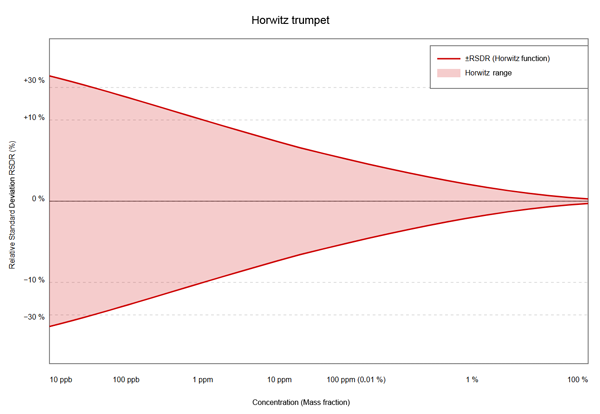
Amazingly, this pattern applies regardless of substance, matrix type, or analytical method.
Mathematically, this relationship can be described by a power function with an unusual exponent (approx. 0.85): RSDR (%) = 2C0.8495 C-1 or 2C-0.1505 [3, 4]. However, at very low concentrations (< 10 ppb), the results deviate from the classic Horwitz function and plateau at a relative standard deviation of 20 – 25%, as they would be largely below the detection limit [4]. Hence, the “Horwitz range” of approximately 10-7 – 10-1 mass fraction can be derived.
The application of the Horwitz function
Even though the Horwitz function is a remarkably good prediction tool for the trend of standard deviations of reproducibility in the corresponding concentration range for food analysis (to which, according to the author, its application is largely limited), this does not allow the conclusion that it should be used uncritically for any validation purposes [5]. A recent paper published this year also shows that newer data from 20 interlaboratory collaborative studies in food analysis from 2011 to 2017 do not follow the Horwitz model... [6]. In this context, it may also be worth referring to the 2012 paper by Thomson, which describes an alternative to the Horwitz function, as the latter is only suitable for individual methods to a limited extent and ignores detection limits [7].
The Horwitz function is widely used in the food, luxury food, veterinary, and environmental areas, as well as in forensic toxicology, e.g., for nicotine analysis in tobacco products, for a wide variety of analysis methods for wine and must, for plastic additives in water samples, for vitamins in dietary supplements, for heavy metal or mycotoxin analysis in animal feed, or for antibiotics in milk or meat... This is quite understandable, as its use is also regulated depending on the area of application, such as for plant protection products by the EU Pesticide Directive or for foodstuffs via the Codex Alimentarius. In the pharmaceutical area, on the other hand, the use of the Horwitz equation is extremely rare. Even though the Horwitz equation was once mentioned for precision in the USP Medicines Compendium Chapter <10>, its current relevance for pharmaceutical method validations seems to me to be quite low, because
- the Horwitz function does not appear in the current relevant validation guidelines and compendial chapters on method validation, and
- the USP Medicines Compendium discontinued already in 2015 [8, 9].
Although a little research turned up a few publications on method validation and a white paper that refer to the Horwitz function [10–17], its fundamental significance and actual relevance in the pharmaceutical area remains to be seen...
The basic observation that precision deteriorates as the analyte concentration decreases, and therefore the idea of choosing correspondingly broader acceptance criteria for lower concentrations for method validations (e.g., for an HPLC method that is used both as assay for the analyte (à 100% level) as well as an impurity method for trace determinations (à LOQ / specification limit)) seems very understandable and reasonable. However, due to the limited data available, I would personally not recommend using the Horwitz model for method validations in the pharmaceutical area.
If anyone disagrees, please leave me a comment - I look forward to the discussion 😉
References
[1] Horwitz W; Kamps LR; Boyer KW, “Quality Assurance in the Analysis of Foods for Trace Constituents”, J. Assoc. Off. Anal. Chem. 1980; 63 (6): 1344-1354
[2] Horwitz W, “Evaluation of Analytical Methods Used for Regulation of Foods and Drugs,” Analytical Chemistry 1982; 54 (1): 67A-76A
[3] Albert R, Horwitz W, “A Heuristic Derivation of the Horwitz Curve”, Anal. Chem. 1997; 69: 789-790
[4] Thompson M, AMC Technical Brief no. 17, (2004) “The amazing Horwitz function”. http://www.rsc.org/pdf/amc/brief17.pdf, accessed on October 15, 2025
[5] Thompson M, “Limitations of the Application of the Horwitz equation: A rebuttal”, Trends in Analytical Chemistry 2007; 26(7): 659-661
[6] Ehling S, Thompson JJ, Schimpf KJ, Pacquette LH, Haselberger PA, “A Contemporary Look at the Precision of Modern Analytical Methods in Food Analysis and the Relevance of the Horwitz Equation”, J AOAC Int. 2025;108(4): 566-571
[7] Thompson M, “The characteristic function, a method-specific alternative to the Horwitz function”, J AOAC Int. 2012; 95(6): 1803-1806
[8] USP Medicines Compendium <10> Assessing Validation Parameters for Reference and Acceptable Procedures - Guideline for Donors / lnstructions for Staff
[9] Schiavetti B, Wynendaele E, De Spiegeleer B, Mbinze GJ, Kalenda N, Marini R, Melotte V, Hasker E, Meessen B, Ravinetto R, Van der Elst J, Mutolo Ngeleka D, “The Quality of Medicines Used in Children and Supplied by Private Pharmaceutical Wholesalers in Kinshasa, Democratic Republic of Congo: A Prospective Survey”. Am J Trop Med Hyg. 2018; 98(3): 894-903
[10] Apostol I, Kelner D, Jiang XG, Huang G, Wypych J, Zhang X, Gastwirt J, Chen K, Fodor S, Hapuarachchi S, Meriage D, Ye F, Poppe L, Szpankowski W, “Uncertainty estimates of purity measurements based on current information: toward a "live validation" of purity methods”, Pharm Res. 2012; 29(12): 3404-3419
[11] Bento D, Borchard G, Gonçalves T, Borges O, “Validation of a new 96-well plate spectrophotometric method for the quantification of compound 48/80 associated with particles”, AAPS PharmSciTech. 2013;14(2): 649-655
[12] Scherer R, Pereira J, Firme J, Lemos M, Lemos M, “Determination of Ciprofloxacin in Pharmaceutical Formulations Using HPLC Method with UV Detection”, Indian J Pharm Sci. 2014; 76(6): 541-544
[13] Kumnerdnon P, Rojsitthisak P, Niwattisaiwong N, Sotanaphun U, Chatchawalsaisin J, Sutanthavibul N, “Validation of an RP-HPLC Method for Quantitative Analysis of Phikud Navakot Extract using the Standard Addition Method”, TJPS 2016; 40(1): 26-31
[14] Surasarang S, Karnpracha C, Boonyapiwa B, “Analytical Method Validation for Testing of Limit of High Molecular Weight Proteins in Filgrastim Biopharmaceutical Products”, IJPHS 2019; 1(1): 12-25
[15] Li W, (2019) “Points to Consider in Quality Control Method Validation and Transfer”, BioProcess International; https://www.bioprocessintl.com/qa-qc/points-to-consider-in-quality-control-method-validation-and-transfer, accessed on October 15, 2025
[16] Quynh Trang NT, Van Hop N, Giang Chau ND, Tran TB, “Simultaneous Determination of Amlodipine, Hydrochlorothiazide, and Valsartan in Pharmaceutical Products by a Combination of Full Spectrum Measurement and Kalman Filter Algorithm”, Advances in Materials Science and Engineering 2019; 1-9 (Article ID 5719651)
[17] Marson BM, Concentino V, Junkert AM, Fachi MM, Vilhena RO, Pontarolo R, “Validation of analytical methods in a pharmaceutical quality system: an overview focused on HPLC methods”, Quim. Nova 2020; 43(8): 1190-1203
