Peak symmetry, asymmetry and their causes in HPLC
In another blog post, we have already discussed the problem of peak broadening under the topic "HPLC: What to do in case of peaks being too broad?” while focussing on symmetrical broad peaks. In this blog post, we will emphasize more on the asymmetric peaks.
What does “Fronting” and “Tailing” mean?
A peak is considered asymmetric when the distance from the start of the peak to the centre (A) and from centre to the end (B) of the peak differs (Fig 1). It is best to measure these distances at about 10% of the peak height. Within asymmetric peaks, there are two possibilities that could exist; Fronting and Tailing. If a peak has a wider front half (distance A) compared to the back half (distance B), it called “fronting” (or “leading”) and vice versa is known as “tailing”. Both effects can be caused from similar, but also from very different reasons and some of the important ones will be discussed here.
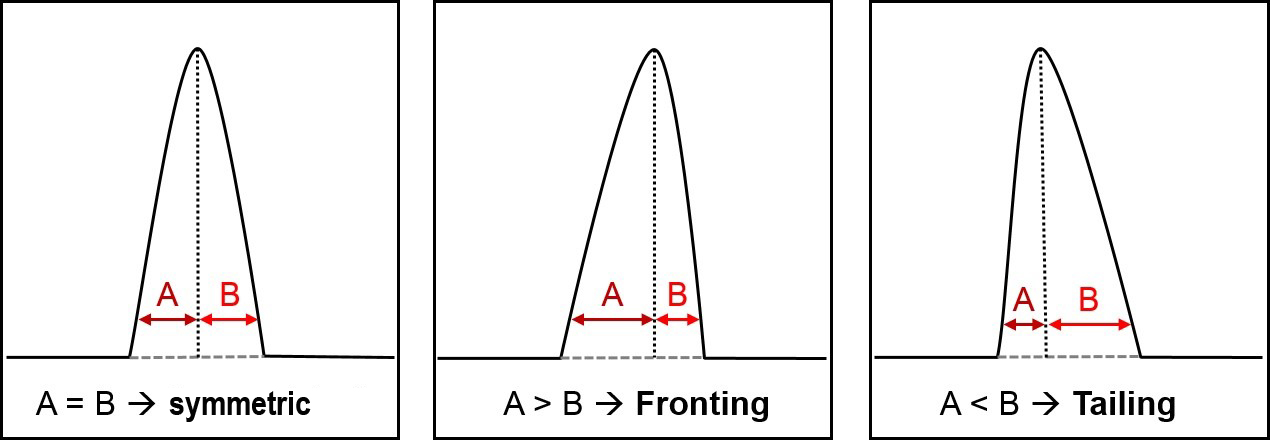
Figure 1: Shows different forms of asymmetric peaks.
“Tailing” may have multifarious reasons
One important aspect to consider when tailing effects are seen consistently is whether it already started at beginning or the effect grew stronger gradually with the increasing use of the column. The answer can be found by comparing an actual chromatogram to an existing older one. This is a simple way to find out and if the recent chromatogram shows significantly more tailing, this is a clear indication of increasing contamination of the HPLC system or simply the aging of the column. As discussed in the earlier post, the maintenance should start by decontaminating the system (e.g. by preparing new solvents) and if required, by replacing the guard column. If the problem persists, the analytical column should be tested on another HPLC system to evaluate the fitness of the column for further analytical runs. If the chromatogram shows strong tailing, the problem is directly linked with the column itself. Depending on the age and the number of samples already analyzed, proper cleaning of the column by rinsing overnight can solve the problem. If the column has been in use for a while, then the column material itself can be the cause of the tailing. Each column has a limited shelf life. Depending on the sample type and HPLC method, this may be the case after five hundred or several thousand samples. Usually, an isocratic system is more column friendly when compared to its gradient counterpart. Moreover, flow rates or temperatures being too high, the use of solvents with higher ionic strength or extreme pH conditions, all contribute towards the aging of the column material.
However, if the tailing was observed from the beginning, the focus must shift from the column to the reagents asit seems to be a chemical problem. In particular, basic substances show peak tailing when overloaded. This means: dilute the sample or reduce the sample volume. But also the pH of the solvent can result in the formation of asymmetric peaks. The cause of a pH tailing or fronting lies in the chemical nature of the sample to be analyzed. Basically you can remember: In an acidic environment, most of the substances are protonated. For basic substances, this means ionization (positive charge, e.g., R-NH3+) while acidic substances are uncharged. In the alkaline range, however, the process is reversed i.e., the substances are correspondingly deprotonated: basic substances are uncharged, acidic substances receive a negative charge (for example R-COO-). If the pH of the mobile phase matches the value of the acid constant, i.e. the pKa value of the sample substance, theoretically half of the molecules of one substance would be charged and the other half uncharged. The charged molecules are more polar and hence, interact less with the apolar reversed phase (RP) column material than the uncharged part. This leads to varied interactions on the column, which in turn can lead to peak broadening. Depending on the distribution between charged and uncharged molecules of a substance the fronting or tailing effect is decided. It should be noted that the proportion of organic solvent in the aqueous buffer also influence the pKa value and thus can affect the ionization. To avoid fronting or tailing due to the pH value, the solvent pH should be set at a value that is distant from the pKa value of the substance to be analyzed.
The tailing described above due to the aging of the column can partly be also influenced by the pH of the eluent. In reversed-phase HPLC, the silanol groups of the silica gel are modified with long carbon chains (e.g. C18). Remaining silanol groups are often endcapped with trimethylsilyl groups to prevent polar or ionic interactions. During the life of a column, these groups can be lost with the release of silanol group through a process called “bleeding”. Thus, positively charged sample constituents can undergo ionic interactions with the silanol groups, which can lead to tailing on aged HPLC columns.
Typical causes of fronting
In fronting, the first thought for an analyst is "overloading." A distinction must be made here between overloading with sample material and overloading with sample volume. Overloading the column with excess material is a common mistake for samples of unknown concentration or mistakes during dilution - especially in case of neutral and acidic samples. This problem can be resolved quickly: simply dilute the sample or inject less sample. Often, this already solves the problem and the sample has just been concentrated. If not, consider analyzing the pH of the eluent.
If the peaks of a chromatogram are flatter and asymmetrically shifted to the front and nearly appear like a double peak (split peak), it is usually because of a too strong solvent. Injecting a sample in a too strong solvent causes the components of the sample to be swept along with the solvent instead of being able to bind properly to the column. Therefore, if possible, the HPLC samples should always be dissolved in the same mobile phase that corresponds to the initial conditions of the HPLC run. For samples that are difficult to dissolve, the volume of the sample to be injected must be reduced to such an extent that the influence of the strong sample solvent on the column is negligible. In principle, however, a larger volume of low concentrated sample dissolved in the mobile phase should be preferred over a small volume of highly concentrated sample prepared in a strong solvent. Another reason for fronting is a too low column temperature. This problem can easily be solved by using a column oven.
Since troubleshooting for HPLC is very time consuming, we hope to be able to help with this blog post and to have pointed out some solution ideas.
