What is GMP?
GMP stands for "good manufacturing practice". In addition to a flawless manufacturing process, GMP ensures quality and proper control of the resulting product. This is important for products that are targeted towards consumers; mainly drugs and their active ingredients, extending to cosmetics, food, and feed.
A faulty manufacturing process of products can have a direct or indirect (poor quality animal feed) effect on the health of the consumer. To prevent this, guidelines for good manufacturing practice have been developed.
GMP and drugs
Good manufacturing practice is very important, especially in pharmaceutical production, as the health of patients is directly linked to the quality of the drug. In this context, two terms are of crucial importance: patient safety and product quality. It goes without saying that the patient should not be harmed in any way by taking the drug (à patient safety), at the same time the actual purpose of healing / relief should be ensured by a high product quality.
Therefore, special guidelines and laws have been established for the manufacture and distribution of pharmaceuticals. In addition to the German Medicinal Products Act (AMG), the German Ordinance on Manufacturing of Medicinal Products and Active Ingredients (AMWHV) and the EU GMP Guideline, the guidelines of the "International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use" (ICH), like the ICH Q2(R1), also contribute here. These guidelines ensure continuous good quality as well as efficacy and safety during the approval process. These basic requirements - quality, efficacy and safety - originate from the fundamental idea of the AMG: "It is the purpose of the present Act to guarantee safety in respect of the trade in medicinal products, ensuring in particular the quality, efficacy and safety of medicinal products in accordance with the following provisions, in the interest of furnishing both human beings and animals with a proper supply of medicinal products." (AMG §1).
Talking about the quality of a drug: it entirely depends on good manufacturing practice. To be able to maintain a good manufacturing practice, the surrounding must be alright as well. In addition to validated methods for manufacturing and quality control, it is mandatory to have suitable premises, qualified equipment and trained personnel. This is known as “Quality management”.
GMP-compliant quality management
A company that wants to adhere to good manufacturing practice must follow some basic steps:
1. The introduction of a document management system helps to manage the load of paperwork that is created. A good manufacturing practice requires proper, methodical, and complete documentation. Anything related to the drug manufacturing process must be routinely documented. This includes the documentation of all manufacturing processes, in-process controls (IPC), and the quality control (QC) of the finished product including its packaging. Incoming goods inspection of the raw materials and primary packaging materials should not be forgotten. Should there nevertheless be deviations in the process, these deviations must also be documented, evaluated and preventive actions must be defined.
2. All instruments and the premises must be examined for their suitability and reliability. This must be documented as well. As we can see by this short summary about some GMP requirements, GMP encompasses a lot. The employees must be thoroughly trained and given simple and precise standard operating procedures (SOP). Such practices not only ensure smooth functioning but also reduce a lot of paperwork due to unnecessary mistakes.
All rooms are qualified, equipment and documentation are running smoothly? Then let’s start - with process validation and in our case much more interesting method validation!
Why is it important to validate methods?
In simple terms: during the validation process the parameters are set, and all steps are documented accurately. Every specific analytical method established as a part of the development process must undergo the process of validation. To be accepted, these methods must show consistent reproducibility and repeatability even when performed by different personnel and at different sites. You can rely on the result. A validated process for manufacturing and validated analytical methods for quality control (during and after manufacturing) is mandatory. The use of validated methods in quality control is an important part of good manufacturing practice. In case of a poor quality of raw materials any manufacturing process doesn’t make sense. The in-process controls, i.e. quality controls at critical steps during the manufacturing process, allow an early detection of potential deviations and thus possibly an interruption in time. In case of deviations in the manufacturing process as well as in case of out of specification results during release analysis or if limit values of IPCs are exceeded, troubleshooting must be initiated to find the root cause, and to prevent further errors and greater loss in the future. Finally, batch release analysis will show if the product is allowed to be sold on the market. Manufacturing of drugs takes place in so-called batches, which is the total amount of all containers (e.g. vials, ampoules or syringes) produced in one working step. The release analysis encompasses a set of different analytical tests defined during the approval of the drug product in order to check the quality of the manufactured batch with regard to potency, purity and identity. Different analytical methods are used depending on the product manufactured.
Where does quality assurance end?
Not before all analyses for quality control of a batch are passed and the manufacturing batch record has been completely checked, this batch of the drug can be released by the responsible person (also known as a qualified person) for sale. For each batch, all controls must be clearly traceable from the starting material to the finished product. Release of the drug to the market does not automatically mean that quality assurance will end. The finished drug product must be able to be stored and transported within the claimed shelf life without loss of quality, which must be proven. Therefore, the transport must be safe and storage at the dealer must be possible and appropriate.
A simplified overview is shown in the following figure:
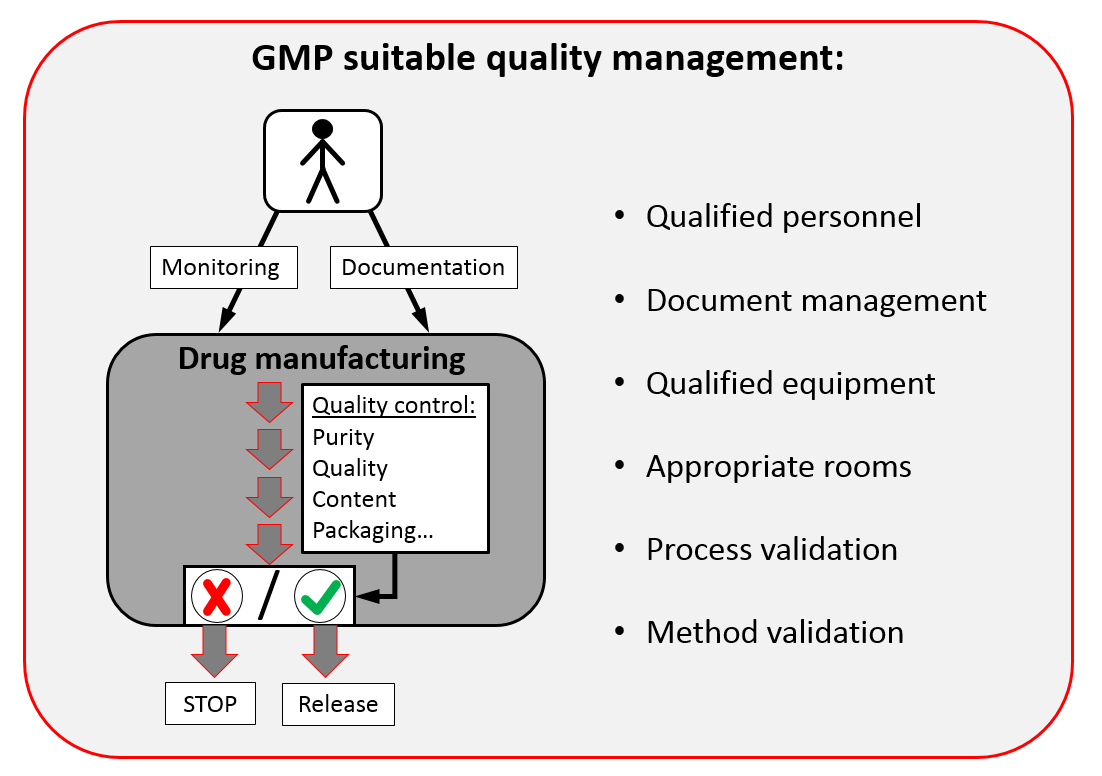
In conclusion, good manufacturing practice can be difficult - but only in compliance with these guidelines we (as consumers) can rely on the quality of drugs.
GMP in the pharmacy
Finally, we’d like to explore the pharmacy’s perspective, as GMP aspects must also be considered here, i.a. in case of drugs produced in the pharmacy as e.g. ointments for skin diseases.
In this context, it should be mentioned that a quality management system is also required for pharmacies. This is to ensure that the customer receives good advice and that all drugs produced in the pharmacy are properly manufactured, adequately tested, correctly stored and can’t be confounded. For this, the processes should be described in standard operating procedures analogously to the industry. Another aspect of good drugs is their microbiological quality. Therefore, the actions defined in the hygiene plan aim to ensure the cleanest possible production areas with low germ loads.
Just like in the industry, the pharmacy is also obliged to perform quality control tests in the lab. This includes, for example, verification of the identity of starting materials to be used, which is often done by near-infrared (NIR) spectroscopy. Also, in-process controls can occur, which have to be taken into account during release of the drug by the pharmacy manager, since usually no final inspections take place.
In addition to regulated storage, documentation also plays an important role in the pharmacy. For example, a written manufacturing and test instruction must specify how the formulation must be prepared and tested, and by completing manufacturing and testing protocols the evidence of the correct manufacturing and the laboratory test results must be provided.
