The HPLC system: simply explained
Not only in university laboratories, but especially in the laboratories of the pharmaceutical industry or of contract research laboratories, HPLC methods play an important role e.g. as purity tests of medicines and their active pharmaceutical ingredient during quality control. Therefore, let's take a closer look at the HPLC system.
High Pressure Liquid Chromatography (HPLC) is a technique widely used to separate various substances in solution. A HPLC system could be thought as an example of a steady flow:
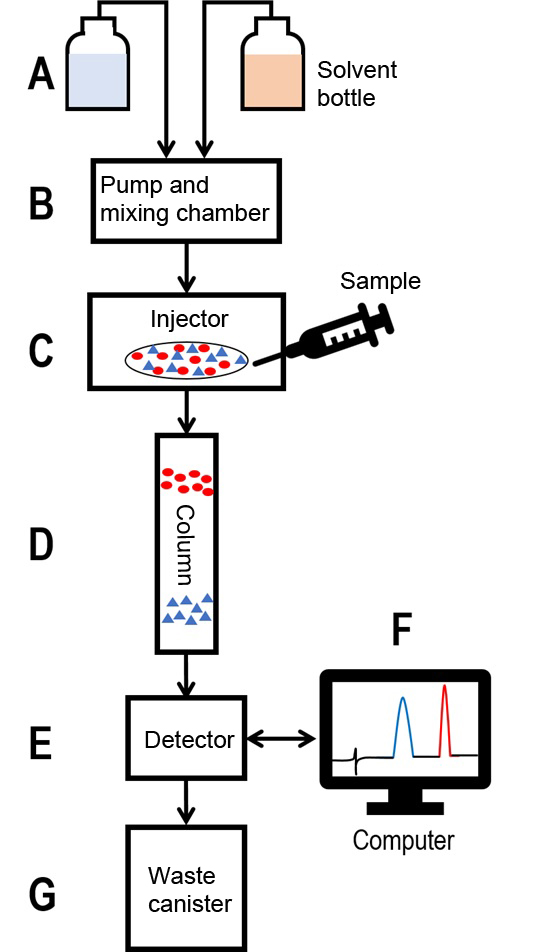
Using a pump, the mobile phase components from their respective reservoirs (A) are transported to a common mixing chamber (B). The mobile phase usually contains a mixture of polar and non-polar components whose concentrations are varied depending on the sample composition. The mixing of the components happens in a pre-determined ratio that is controlled by the pump. From the mixing chamber, the mobile phase mixture goes to the sample injector (C). The sample injector can be manual (manual injection valve) or fully automated (autosampler). Via a separate capillary tube (sample loop), the injector introduces the sample to be analyzed to the mobile phase solvent and the sample in turn is entrained by the solvent flow. The sample flowing with the mobile phase now encounters the HPLC column (D).
The columns are packed with adsorbent particles (stationary phase), which are different dependent on the column type. During the passage through the column, each component in the sample, because of its different physical/chemical properties, interacts with the column material in a slightly different manner. One commonly used column material is the reversed phase (RP). It consists of silica gel particles with long hydrocarbon chains (C8 or 18). Due to the presence of hydrocarbon chains, this column material has a non-polar characteristic. Highly non-polar sample components therefore interact strongly with the column material and get caught. The polar constituents on the other hand pass through the column largely because of limited interaction with the stationary phase.
Back in the mixing chamber (B), the ratio of the mobile phase solvents can now be changed in a way so that the sample components bound to the column are washed away gradually. In general, this is achieved by creating a linear gradient between two components. In our example with RP as stationary phase, changing the ratio of solvents from polar (e.g., water, light blue) to nonpolar (e.g., acetonitrile, light red) is sufficient. The more non-polar the mobile phase mixture becomes, the sooner the non-polar sample components are detached from the column particles: with the weakly bound components eluting first.
The continuous stream of solvent then passes through the detector (E), which detects the various dissolved sample analytes. Depending on the application, there are a variety of available detectors. The simplest would be a UV/Vis detector, which measures the absorbance of the sample constituents at a specified wavelength. The measurement is recorded using a computer program (F) and a chromatogram is obtained in which ideally, every individual sample component produces an absorbance peak. The chromatogram provides the retention time, this is the time from injection to detection, of the respective peak. In our example, polar constituents (blue) have a significantly lower retention time than their nonpolar (red) counterparts. Knowing the sample components, it is possible to identify the peaks by comparing the retention times and absorbance spectra with known standard substances. Identification of unknown substances with this method, however, is far more complex. For that, one needs additional downstream analytical methods to study, for example, the mass (mass spectrometer) or structure (nuclear magnetic resonance, NMR). For this purpose, individual HPLC fractions can be collected using a fraction collector and subsequently analyzed further. Recent technological advancements have allowed direct coupling of the mass spectrometer to the HPLC system (LC-MS coupling, like in this example), allowing the chromatographically separated sample substances to be directly investigated regarding their masses. This method of detection has gained extreme popularity because the individual capabilities of each technique are enhanced synergistically. The final step of the HPLC, as in all other methods, includes the disposal of the mobile phase eluates into a designated waste container.
Futher HPLC blog articles on topics such as HPLC pressure differences, too broad peaks, peak symmetry in general, increasing sensitivity and method optimization can be found here by clicking on the respective term.